MDM2 inhibition by small molecules as a means of restoring p53 function has shown clinical activity against acute myeloid leukemia (AML) (Andreeff, Clin Cancer Res 2015). However, we and others have found increased variant allele frequencies (VAFs) of TP53 mutations in AML cells after treatment with MDM2 inhibitors, either as monotherapy or in combination with other agents (Daver ASH 2019), which suggests that MDM2 inhibition selects preexisting clones or generates de novo clones with TP53 mutations.
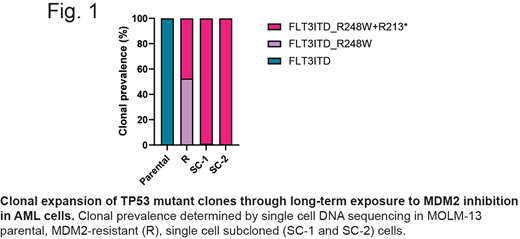
We performed a long-term culture of AML cells (MOLM-13, an AML cell line with wild-type p53 and FLT3-ITD) treated with increasing concentrations of an MDM2 inhibitor idasanutlin (up to 320 nM, less than 10% concentration of Cmax) (Selleck). We obtained MDM2 inhibitor-resistant (R) AML cells after 96 days of the drug exposure and found that the resistant cells harbor hotspot TP53 p.R248W (R248W) mutation. We next isolated single cell clones from MOLM-13 R cells by limiting dilution, and obtained twelve subclones (subclones #1-12 in order of development). All clones carried the same R248W mutation. To determine clonal patterns of these cells, we performed single cell DNA sequencing (scDNAseq) of MOLM-13 parental, R and subclone #1 and #2 (SC1 and SC2) cells using the MissionBio Tapestry system covering 125 amplicons of 19 genes frequently mutated in AML. scDNAseq identified FLT3-ITD mutations in all cells analyzed, as expected. In the parental cells we identified only 0.02% cells (1/ 5,240) with the identical R248W mutation found in MOLM-13 R cells. MOLM-13 R cells had only 0.6% of wild-type TP53 cells, 51% carrying R248W only, and 43% R248W/R213* mutation (R248W/R213*). SC1 and SC2 cells had 1% and 99% of R248W and R248W/R213* clones, respectively (Fig.1). Seven other mutations were detected by scDNAseq. Results suggest that MDM2 inhibition can accelerate the selection of TP53-mutant AML cells in vitro. Of note, the parental cells had remained mostly p53 wild-type, where the subclone with mutant R248W did not have a growth advantage over other cells.
Next we analyzed patient samples enrolled in the phase 1 clinical trial (NCT02319369) for the MDM2 inhibitor milademetan (DS-3032b; Daiichi-Sankyo) in relapsed/refractory AML or high-risk MDS patients. Fifty seven patients were treated with single agent milademetan in the study. All but one patient had wild-type TP53 as determined by NGS at baseline. One patient (1.8%) had a TP53 p.R213* mutation at baseline with VAF of 91%. Four patients (7%) developed different TP53 mutations (R248Q, R248W, P250fs, V122fs and V274L), with increasing VAFs that were not detected at baseline to 19% average, ranging from 11% to 28% post treatment, One patient (anonymized ID 1001-1005) developed both, R248Q and R248W mutations, detected at cycle 2 day1 (C2D1, day 29). The pre-existing R213* mutation detected in one patient persisted with increased VAF after treatment (91% to 100%). To detect p53 mutations with higher sensitivity than NGS, we performed droplet digital PCR (ddPCR) for R248W/Q and R273H in samples from two patients. ddPCR detected 0.46% and 0.62% of R248Q and R248W mutations, respectively, at baseline, which were not detected by NGS, with increased VAFs of 18.2 and 27.6% at C2D1, respectively. ddPCR detected additional R273H mutations with VAFs of 0.08% and 0.18% at baseline, and 2.2% and 2.6% in these patients at C2D1, respectively.
Conclusion: Data suggest that MDM2 inhibition selects rare AML subpopulations with TP53 mutations and careful monitoring of patients treated with MDM2 inhibitors with sensitive methods to detect TP53 mutant clones is warranted. This finding also points to the need to develop strategies to prevent/suppress these clones early on.
Kumar:Daiichi-Sankyo Inc.: Current Employment. Patel:Daiichi-Sankyo Inc.: Current Employment. Dos Santos:Daiichi Sankyo, Inc.: Current Employment. DiNardo:Daiichi Sankyo: Consultancy, Honoraria, Research Funding; Novartis: Consultancy; ImmuneOnc: Honoraria; Calithera: Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Notable Labs: Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Honoraria, Research Funding; MedImmune: Honoraria; Jazz: Honoraria; Agios: Consultancy, Honoraria, Research Funding; Takeda: Honoraria; Syros: Honoraria. Andreeff:Daiichi-Sankyo; Jazz Pharmaceuticals; Celgene; Amgen; AstraZeneca; 6 Dimensions Capital: Consultancy; Daiichi-Sankyo; Breast Cancer Research Foundation; CPRIT; NIH/NCI; Amgen; AstraZeneca: Research Funding; Centre for Drug Research & Development; Cancer UK; NCI-CTEP; German Research Council; Leukemia Lymphoma Foundation (LLS); NCI-RDCRN (Rare Disease Clin Network); CLL Founcdation; BioLineRx; SentiBio; Aptose Biosciences, Inc: Membership on an entity's Board of Directors or advisory committees; Amgen: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal